Liberia Enters Carbon Market
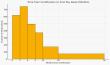
The Liberian Environmental Protection Agency (EPA) is leading the country's entry into the carbon market, aiming to sell carbon credits to companies and individuals seeking to offset their emissions. Dr. Emmanuel Yarpolowo, EPA Executive Director, …