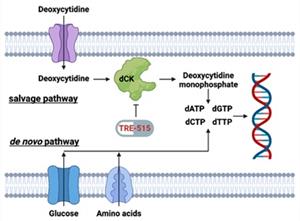
Biotech Stocks Facing FDA Decision In August 2025
As July draws to a close, it's time to take a look at what's in store in the health and wellness space over the upcoming weeks. August, the last month of summer, includes several health observances, such as National Immunization Awareness Month, …