New drug candidates for nerve pain and ischemic disease
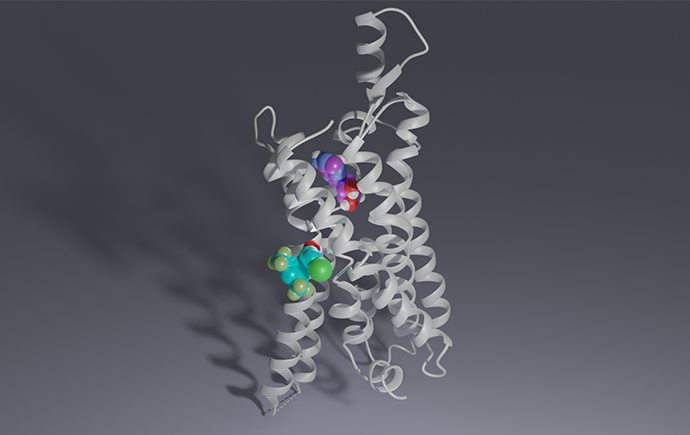
Funder: Information available in the paper: https://www.pnas.org/doi/10.1073/pnas.2421687122 From: Monash University Scientists have discovered novel drug candidates which could ultimately lead to new effective treatments for conditions …