FDA approves updated label for Lilly's Kisunla (donanemab-azbt) with new dosing in early symptomatic Alzheimer's disease
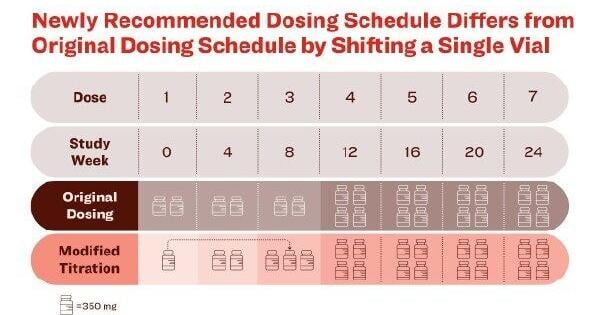
The newly recommended dosing schedule significantly lowered ARIA-E rates compared to the original dosing schedule, adding to the established safety profile of the treatment INDIANAPOLIS, July 9, 2025 /PRNewswire/ -- Eli Lilly and Company (NYSE: LLY …


