Koios Medical Announces FDA Clearance of Next Generation Koios DS Version 3.6 SmartUltrasoundTM Software
Next generation "AI Engine" combines diagnostic precision with workflow optimization now cleared for use in the US and beyond.
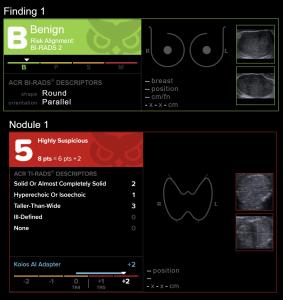
Version 3.6 builds on the software’s already widely proven capabilities, incorporating cutting-edge algorithms and user-centric design enhancements. These updates deliver improved interpretation precision across a wide range of lesion types and image quality, more precise diagnostic capabilities, and further streamlined integration into clinical workflows, eliminating work while empowering healthcare professionals with faster and more reliable insights.
“We are proud to achieve yet another FDA clearance for SmartUltrasoundTM Software Version 3.6, underscoring our dedication to pushing the boundaries of innovation in medical imaging,” said Chad McClennan, President & CEO, Koios Medical. “This milestone represents our commitment to supporting clinicians with solutions that enhance diagnostic confidence and improve patient outcomes especially at a time when physicians and sonographers are in increasingly short supply. Our deepest thanks go out to the hundreds of physicians around the world regularly providing input that fuels our culture of innovation and improvement.”
The new features in Version 3.6 include:
- Enhanced Imaging Algorithms: Delivering greater detail and accuracy for a wide range of common to complex diagnostic cases.
- Optimized Workflow Tools: Reducing time required on image interpretation, classification and reporting enabling more efficient patient throughput.
- Improved Compatibility & Exportability: Seamless integration with a broader range of ultrasound devices, PACS workstations and reporting systems.
Koios DS SmartUltrasound Software Version 3.6 has undergone rigorous clinical validation to ensure it meets the highest standards for safety and performance. Its user-friendly interface and intelligent automation capabilities make it a trusted choice for healthcare facilities ranging from community clinics and imaging centers to major hospital systems and cancer centers.
“This most recent version is the culmination of over two years of machine learning research and collection, curation and augmentation of our training datasets to reflect the widest possible range of images and anomalies found in everyday clinical practice. With our dataset, spanning a wide variety of clinical sources and ultrasound manufacturers, we have developed increasingly robust and powerful models, proven to elevate human performance to superhuman levels of consistency and accuracy.” says Ajit Jairaj, VP Research & Development.
This clearance marks Koios Medical’s continued leadership in the field of smart imaging technology. Building on the success of its previous FDA-cleared innovations, the company remains dedicated to advancing diagnostic accuracy while streamlining clinical operations.
“We are finally seeing unrivaled accuracy. Even more cancers found early, while still treatable, while significantly reducing false positives, biopsy procedures and even eliminating stressful, costly and unnecessary follow-up of benign conditions. The promise of AI is now the reality. Our goal all along has been to empower physicians and sonographers with solutions that give them back precious time while ensuring they can practice at their very best every day, with every patient,” says McClennan.
Koios DS SmartUltrasound Software Version 3.6 will be available for deployment beginning December 1st, coinciding with the Radiological Society of North America (RSNA) annual conference at McCormick Place in Chicago. For more information visit www.koiosmedical.com.
Tonja Haugh
Koios Medical, Inc
+1 716-574-2165
email us here
Visit us on social media:
X
LinkedIn
Instagram
Distribution channels: Healthcare & Pharmaceuticals Industry, IT Industry, Science, Technology, World & Regional
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release