The Independent's journalism is supported by our readers. When you purchase through links on our site, we may earn commission.
Owlet stops selling baby-monitoring sock after FDA warning
Company noted that the FDA had not identified any safety concerns with product
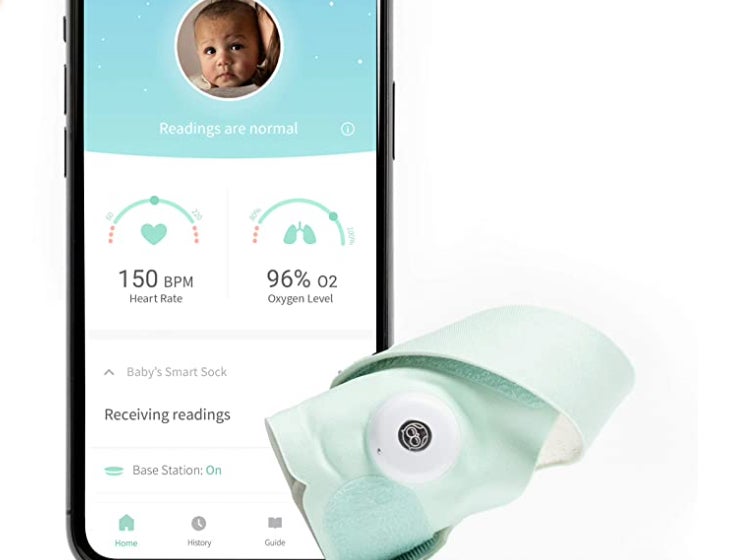
A company that sells baby-monitoring socks has discontinued the product and removed it from its website after a letter from the Food and Drug Administration.
Last month, the FDA issued a warning letter in which it stated that the Smart Sock, sold by Owlet, was actually a medical device, and that the company had been selling the socks without the FDA’s “marketing approval, clearance, or authorisation”.
According to the FDA, the products, which track a baby’s oxygen levels and heart rate, are intended for use “in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body”.
In the letter, the FDA also noted that the baby product company would have to obtain approval or clearance for the device to continue legally marketing it.
This week, Owlet announced in a letter to its own website that it would no longer be selling the Smart Sock products.
In its response, the company acknowledged that the FDA’s letter “did not identify any safety concerns about the Smart Sock,” but rather asserted that the product should be classified as a medical device.
According to the company, based on the FDA’s warning letter, it now plans to pursue “marketing authorisation from the FDA” for the heart rate and oxygen features.
Owlet also said it plans to continue to support its current customers, before assuring those who have purchased the product that there will not be “any change to your product’s functionality”. According to the company, the change will only affect customers in the US, with Owlet confirming that “no other countries or regions are affected by this”.
“With over one million babies monitored, we are extremely proud of the innovation and technology Owlet has delivered. We will continue to stay focused on our mission and are cooperating with the FDA so we can continue to provide sleep monitoring products and solutions to parents and babies,” the company said.
The announcement that the company will no longer be selling the product has been met with mixed responses on social media, where many parents have expressed their disappointment and dismay over the FDA’s warning.
“Wait the @owletbabycare Smart Sock is being pulled? It’s my fav baby gift to give to people! It gave me SO MUCH peace of mind and YES it actually alerted me when my three-day-old baby stopped breathing, sending us to the ICU for days in 2020,” one person tweeted.
Another said: “I love the Smart Sock I don’t know how I would have gotten through the newborn stage without it.”
Others have urged people to sign a Change.org petition in an effort to “fight the FDA” and “keep the Owlet Smart Sock on the market,” with the petition gaining more than 118,000 signatures as of Wednesday.
The Independent has contacted Owlet for comment.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies